Today I decided to expand a bit on the National Drug Codes (NDC) from the FDA, and how the Defense Health Agency (DHA) decided to proceed, MeDiCaLly.
So these are the lot numbers provided in the Dear Health Care Professional Letter, the alleged BLA approved lots, BLA compliant. Whatever. It is a fake term so it does not deserve my respect.

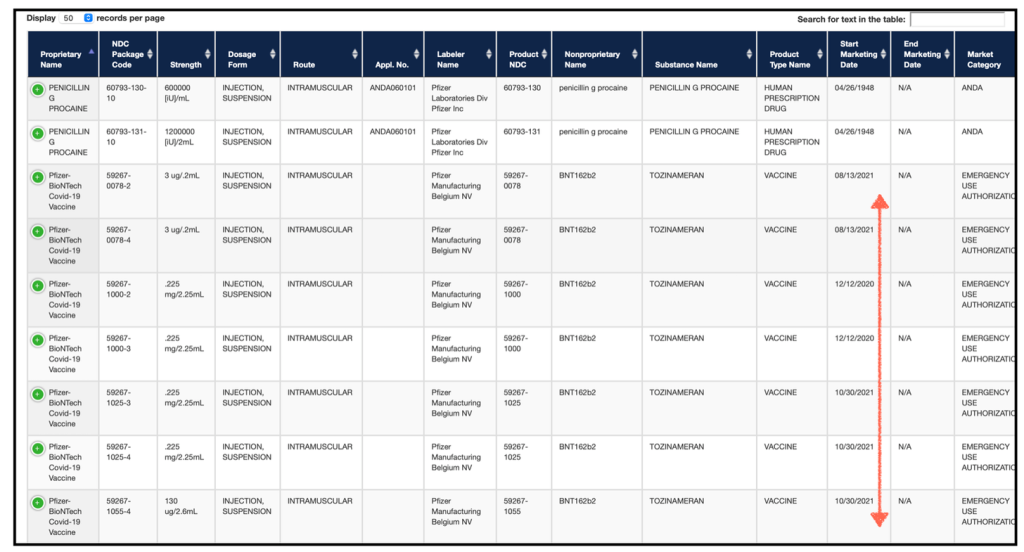
I went ahead an added the corresponding NDC’s that the CDC Immunization database associates with these lots numbers, since the CDC is basically running the Covid 19 immunization program, they are the premier source.
Here is the full list with lots, manufacture dates, expiration dates and NDCs, in case you want to see for yourself.
Alright, so all of these codes are legally designated by the FDA, and really anywhere else, as EMERGENCY USE. So it remains a bit confusing to me that they can be both an emergency use product and an approved product at the exact same time. Voodoo. Keep in mind, these codes are not broken down by lot numbers with noted exceptions. There are no exceptions. One product cannot be subject to two legal regimes (authorized and approved). The news alert here is: that is illegal.
A Congressional Report from September 29, 2021 breaks it down this way:
Why did FDA refer to Comirnaty and the Pfizer-BioNTech vaccine as “legally distinct”?
In the EUA, FDA states that the Comirnaty and Pfizer-BioNTech vaccines “are legally distinct with certain differences that do not impact safety or effectiveness.” While the Comirnaty and Pfizer-BioNTech vaccines have the same formulation, they are legally allowed to be marketed and used pursuant to different legal authorities. Specifically, Comirnaty is licensed pursuant to a BLA issued under the PHS Act (42 U.S.C. §262). The Pfizer-BioNTech vaccine is authorized for emergency use pursuant to the Federal Food, Drug, and Cosmetic (FD&C) Act (21 U.S.C. §360bbb-3).
Each product must be manufactured, labeled, marketed, distributed, and administered in accordance with the requirements of the legal regime under which it was approved or authorized. These requirements may differ in a number of ways. For example, under the EUA, the Pfizer- BioNTech vaccine must be accompanied by fact sheets for the vaccine administrator and recipient informing them, among other things, of the product’s emergency authorization, known and anticipated risks and benefits, and the right to decline the vaccine. Comirnaty need not be accompanied by this information if it is being administered pursuant to the BLA rather than the EUA; instead, the PHS Act and other FDA regulatory labeling requirements apply.
As another example, the Pfizer-BioNTech vaccine may be manufactured only at facilities identified and agreed upon in Pfizer’s EUA request, must be distributed directly by Pfizer or through authorized distributors to emergency response stakeholders (as defined in the EUA) as directed by the U.S. government, and must be administered by vaccination providers (as defined in the EUA) only to individuals 12 years of age and older in accordance with the uses authorized by the EUA. These limitations do not apply to Comirnaty vaccines manufactured and distributed pursuant to the BLA; instead, the PHS Act and FD&C Act requirements apply. Comirnaty may be manufactured only at facilities identified and approved in the BLA.”
Pretty cut and dry in my opinion. And oh! look at those codes category.
Now, the DoD has some lawyers going to court when called upon (which is a lot lately), and they have said, under oath, that the EUA product needs to stay in play because there isn’t an approval for those 12-15. Well, that’s incorrect. An approved product can be granted an EUA for unapproved indications, in this case for those 12-15. So, that was a bald (bold?) faced lie. I will demonstrate.

Now we know BLA Compliant is a scam and that the licensed product could cover all EUA indications. But that’s just the law right? What is one minor inconvenient detail when you are trampling the rights of informed consent military wide? Small hurdles, but no worries, the Defense Health Agency is ready to play ball with the legal and medical deception. The lot numbers on this next image may look familiar.


What the hell guys? You are telling me no one in the DHA was like “y’all know this is illegal right?” Good thing SpikeVax was approved so we can do it all over again.

And just so we are on the same page, and this particular page is straight from the CDC. It’s still active. It is still being updated. Sorry for the mashup, but it is easier to look at. All I know is, I know they’re lying and they know they’re lying.

It’s Friday. Gotta get down on Friday. Everybody’s lookin forward to the weekend.